Translation of Medical Device Labeling
Medical device labeling must be translated with absolute precision. Errors or ambiguities can lead to regulatory delays, product recalls, or patient safety risks. GTS provides professional, certified translation of medical device labeling for global markets, ensuring linguistic accuracy and regulatory consistency across all required languages.
Medical device labeling includes all text and symbols appearing on the device itself, packaging, and accompanying documentation.
These materials typically contain:
- Usage instructions and warnings
- Storage and handling conditions
- Environmental limits (temperature, humidity, pressure)
- Standardized symbols and pictograms
- Regulatory and safety information
Accurate translation is essential to ensure that end users, healthcare professionals, and regulators fully understand the device requirements in each target market.
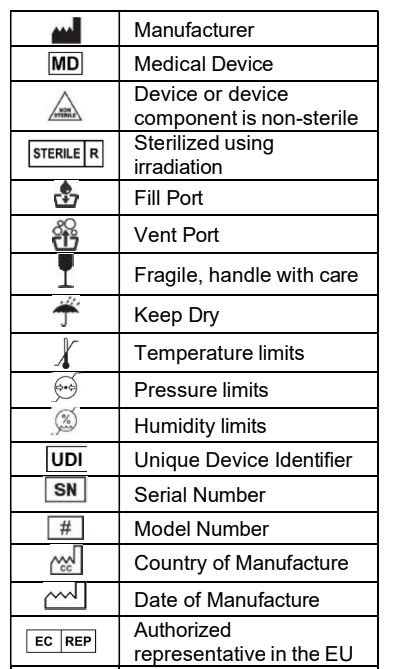
Medical Device Labeling Symbols & ISO 15223-1
Medical device labeling often relies on standardized symbols rather than full text. These symbols are governed by ISO 15223-1 and must be interpreted and documented correctly across languages.
Regulatory Language Requirements for Labels
Medical device regulations require IFUs and labeling to be available in the official language(s) of each target market. Examples include:
- EU MDR requirements for Member State languages
- National authority language mandates
- Distributor-specific language obligations
- Local labeling and packaging rules
Failure to provide compliant translations can delay product registration or market entry. GTS works with experienced medical translators who are familiar with regulatory expectations and terminology conventions across global markets.
Our Approach to Translating Regulated Labeling Content
GTS provides professional medical device translation services under an ISO 17100-certified quality framework. Our services include:
- Translation of IFUs, labels, and packaging content
- Symbol legends and regulatory text translation
- Terminology consistency across product lines
- Multilingual IFU production for global distribution
- Secure file handling and controlled workflows
All translations are performed by qualified human translators with subject-matter expertise in medical and technical content. Whether you are launching a new product or updating existing documentation, we help ensure consistency and accuracy across all languages.
Customers Trust GTS
Medical device labeling and IFUs contain regulated content that must remain consistent across languages, formats, and markets. Our medical translation workflows are designed specifically for this type of regulatory documentation.
- Experience with regulated labeling content
- ISO 17100 workflows for medical translation
- Terminology consistency across product lines
- Secure handling of controlled documentation
Our translators understand how labeling symbols interact with IFU text and regulatory documentation, ensuring nothing is lost or misinterpreted in translation.
Get a Quote for Medical Device IFU Translation
GET PRICE QUOTE FOR MEDICAL DEVICE TRANSLATION SERVICES
EU Translation of common medical device label symbols
As a free service to our clients, we would like to provide you with translations of the most common labels used in medical device Instructions for Use (IFUs). The download link is provided at the end of this page.
Click the following link to download a PDF file with translations of the ISO 15223-1:2016 label texts.

